
It is located in Group 16 as a non metal yellow solid at room temperature, it is brittle. Whilst Sulfur does not smell itself the compounds that it creates such as Hydrogen Sulfide produces a smell similar to that of rotten eggs. Sulfur is used in the vulcanisation of rubber and in the production of gunpowder, its most important use is in the production of Sulphuric acid in the contact process. Sulfur Data Sulfur Atomic Radius State at 20 C Solid Uses Used in matches, gunpowder, medicines, rubber and pesticides, dyes and insecticides. In the case of Sulfur There are cool facts about Sulfur that most don't know about. It is a non-metallic, yellowish, odorless, flavorless element. Note: Learn more about the atomic radius here.

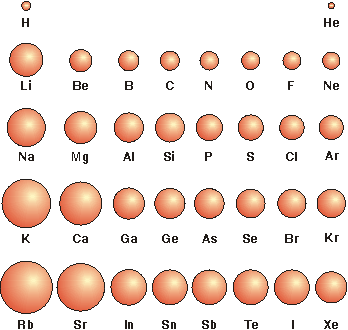
Sulfur however has been known since ancient times due to its elemental presence in volcanoes. Sulfur has the atomic number 16 with an atomic weight of 32.064 g/mol. The bigger the atomic radii, the weaker the electronegativity. The atomic radius of a chemical element is usually measured by the distance from the center of the nucleus to the boundary of the outermost layer of electrons.
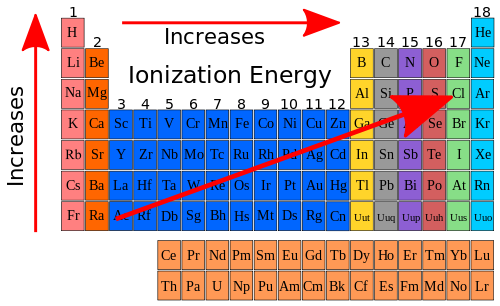
Sulfur exists as allotropes (similar formulas but different structures) of a rhombic and monoclinic Sulphur crystals. The higher the atomic number, the stronger the electronegativity. It was discovered by Antoine Lavoisier in 1789 but in 1823 by the German Chemist Eilhard Mitscherlich obtained Sulfur crystals from cooling molten sulfur. Sulfur is a abundant non metal that makes up 3% of the earth’s mass. It is brittle and crystalline at room temperature. Sulfur (S) a yellow solid non metal in Group 16 of the periodic table. Sulfur dioxide (SO 2) is produced when unpurified oil & coal are burned, and due to this process sulfur dioxide can enter the atmosphere, where it reacts with oxygen to produce H 2 SO 4 and H 2 SO 3, which causes acid rain. The van der Waals radius (also known as the nonbonding atomic radius) is the radius of an atom which is not bonded to other atoms this is determined by measuring the distance between atomic nuclei which are in direct but nonbonding contact with each other in a crystal lattice.


 0 kommentar(er)
0 kommentar(er)
